Body weight and
sympathetic activity
In experimental and clinical studies, it
has been shown that overweight persons exhibit an increased activity of
the
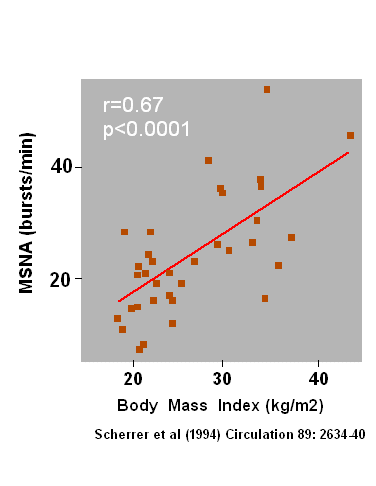
sympathetic nervous system. It was reported by Scherrer et al.
(1) that skeletal muscle sympathetic nerve activity correlates
with body mass
index, whereby the correlation already starts with a body mass index of
approx. 23. Thus, a raised sympathetic activity is not solely a problem
of obese persons, i.e. BMI greater 30, but is present already in
moderately overweight persons.
It might be asked why
the body responds to a rise in body weight by increasing sympathetic
activity. It can be speculated that during evolution, the body
had to adjust to periods of food shortage by reducing energy
expenditure by downregulating
sympathetic activity, i.e. bradycardia and reduced basal metabolism.
Only
in recent decades, living conditions have changed in a manner which
brings
the body in a situation where he has to deal with a chronically raised
caloric
intake. It appears that the body uses the same mechanisms acquired
during
centuries of intermittent food shortage and raises the sympathetic
activity
in parallel with the caloric intake. One of the consequences of this
chronically
increased sympathetic activity is a high incidence of hypertension and
insulin resistance and the consecutive diabetes mellitus type-2.
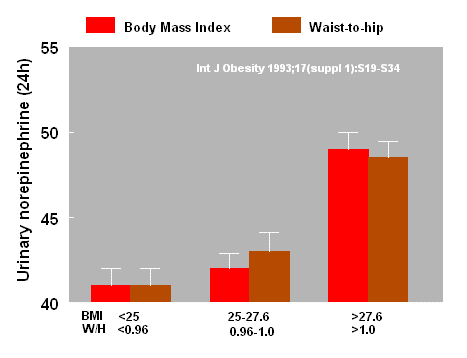
 It has also been reported that urinary
catecholamine metabolites are increased in parallel with the rise in
body weight judged either by the body mass index (BMI) or the
waist-to-hip ratio (2).
It has also been reported that urinary
catecholamine metabolites are increased in parallel with the rise in
body weight judged either by the body mass index (BMI) or the
waist-to-hip ratio (2).
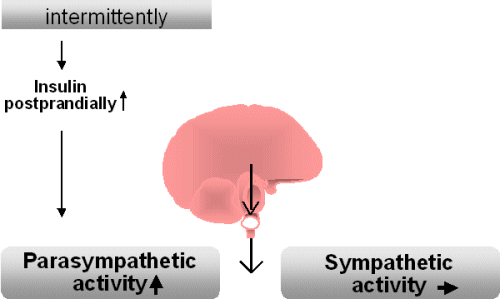
The fact that an increase in
body weight is associated with a raised sympathetic activity may be
surprising. Shortly after food intake, sympathetic activity is either
unchanged or reduced
which is associated with a raised parasympathetic activity. This
postprandial
state is characterized by the absorption of nutrients and is dominated
by
the parasympathetic system activity.
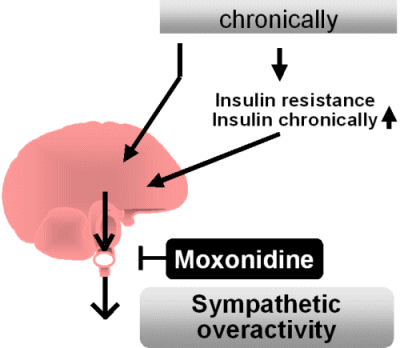
By contrast, a chronically
increased caloric intake leads to a raised sympathetic activity which -
when referred to the physical activity - has to be rated as sympathetic
overactivity.
It appears that the parasympathetic activity remains unaltered. This
chronic rise in sympathetic activity has various adverse effects. It is
currently not known how this increase in centrally mediated sympathetic
overactivity is triggered. According to one hypothesis, a chronically
high caloric intake leads to insulin resistance which requires
hyperinsulinemia to maintain
normal blood glucose levels. The hyperinsulinemia is expected to
stimulate
sympathetic acitivity. It is, however, also possible that a high
caloric
intake directly stimulates sympathetic outflow of the brain.
Since BMI,
waist-to-hip ratio or abdominal visceral fat are closely linked to
sympathetic outflow of the brain, a drug which reduces an increased
sympathetic outflow of the brain represents a logical therapy for
interfering with overweight associated diseases such as hypertension
and insulin resistance. Moxonidine is currently the only drug which
reduces sympathetic outflow with minimal adverse side effects.
1. Scherrer U, Randin
D, Tappy L, Vollenweider P, Jequier E, Nicod P: Body fat and
sympathetic nerve activity in healthy subjects. Circulation
1994;89:2634-2640




